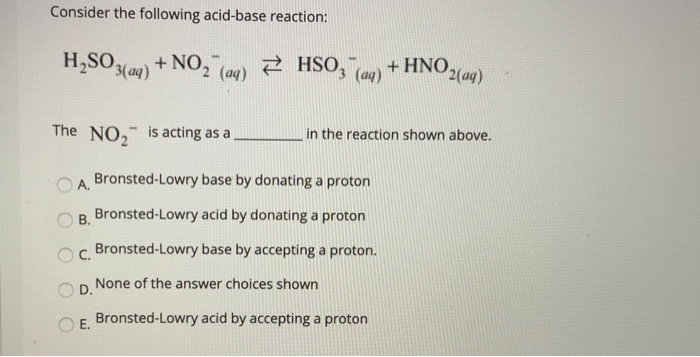
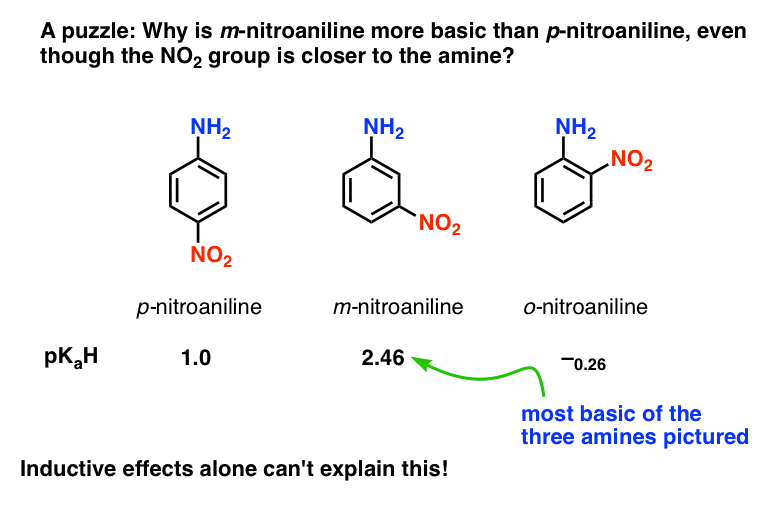
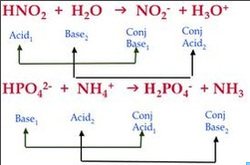
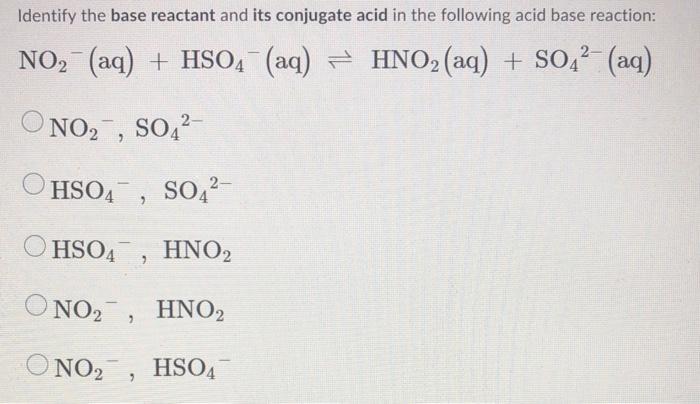
SOLVED: 19. Which of the following is not conjugate acid-base pair? A) Hz0,HO B) H2O, Hzot C) HSO4 , HzS04 D) -OH,02- E) NO3 , NO2"

Yoshi Baza hybrydowa do paznokci Rubber Base UV Hybrid No2 10ml Rubber Base No2 | Paznokcie \ Bazy i topy Promocje Promocja - Kosmetyki Plus
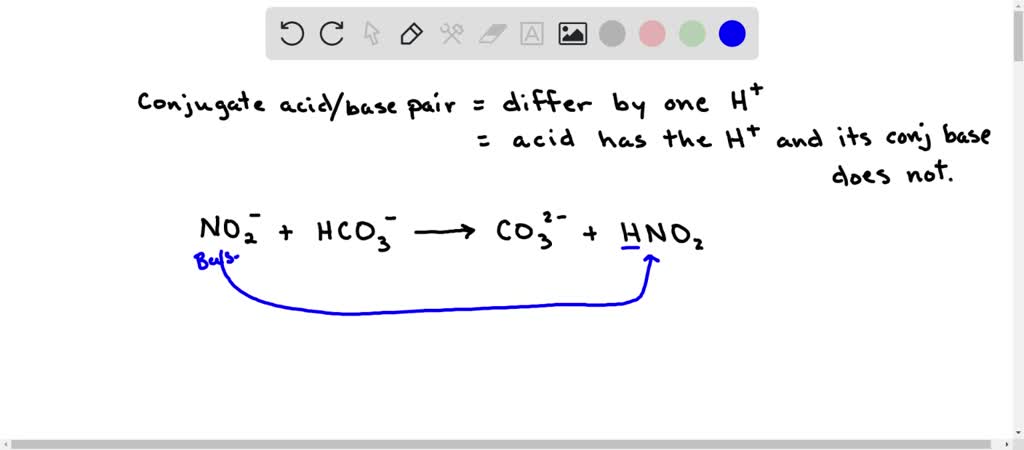
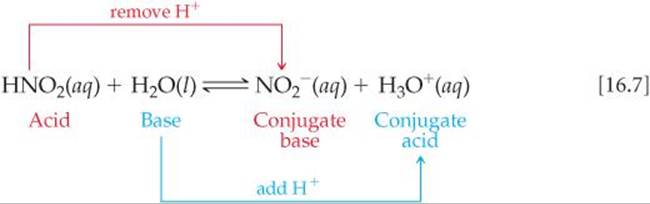
SOLVED: Consider the following reaction: NO2 - + HCO3 - ⇌ CO3 2- + HNO2 Identify the acid, base, conjugate acid and conjugate base.

OneClass: 26 Which set is a conjugate acid/base pair? Marks: 1 Choose one answer a. HNO3 NO2 b. HNO2 ...
Sensors | Free Full-Text | Free-Base Carboxyphenyl Porphyrin Films Using a TiO2 Columnar Matrix: Characterization and Application as NO2 Sensors

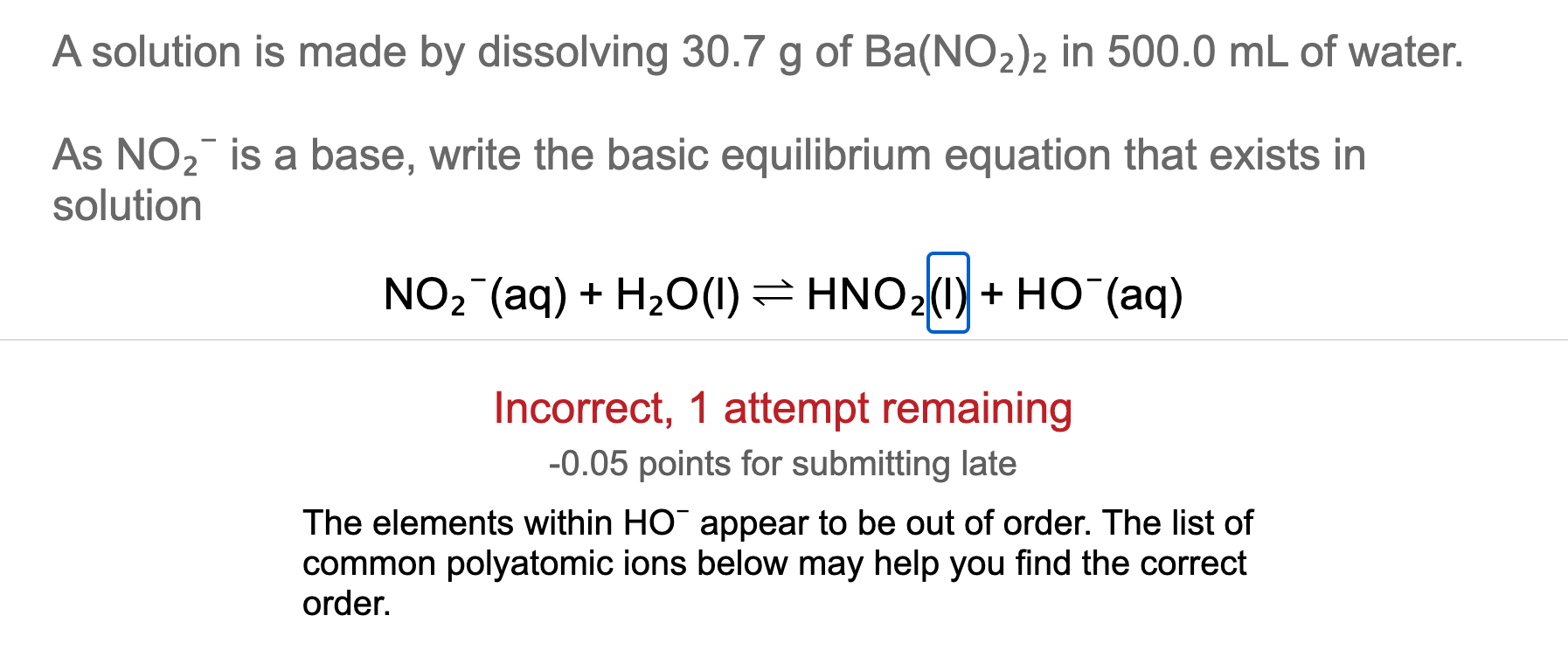
1. What is the Bronsted Acid in the following equation: * NO2- +H2O HNO2 + OH- **a. NO2- **b. H2O **c. HNO2 **d. OH- 2. What is the Bronsted base in the

organic chemistry - What are the resulting conjugate acid and base of phenol and 4-nitrophenol - Chemistry Stack Exchange