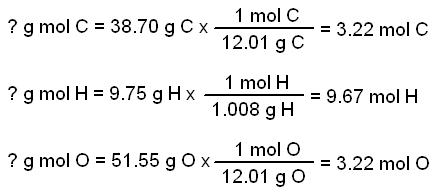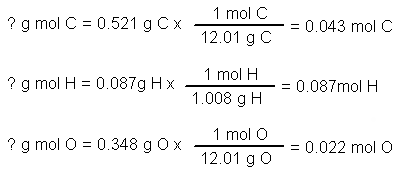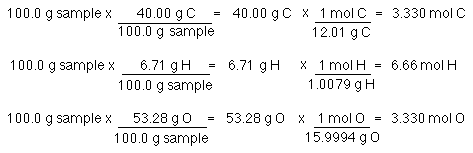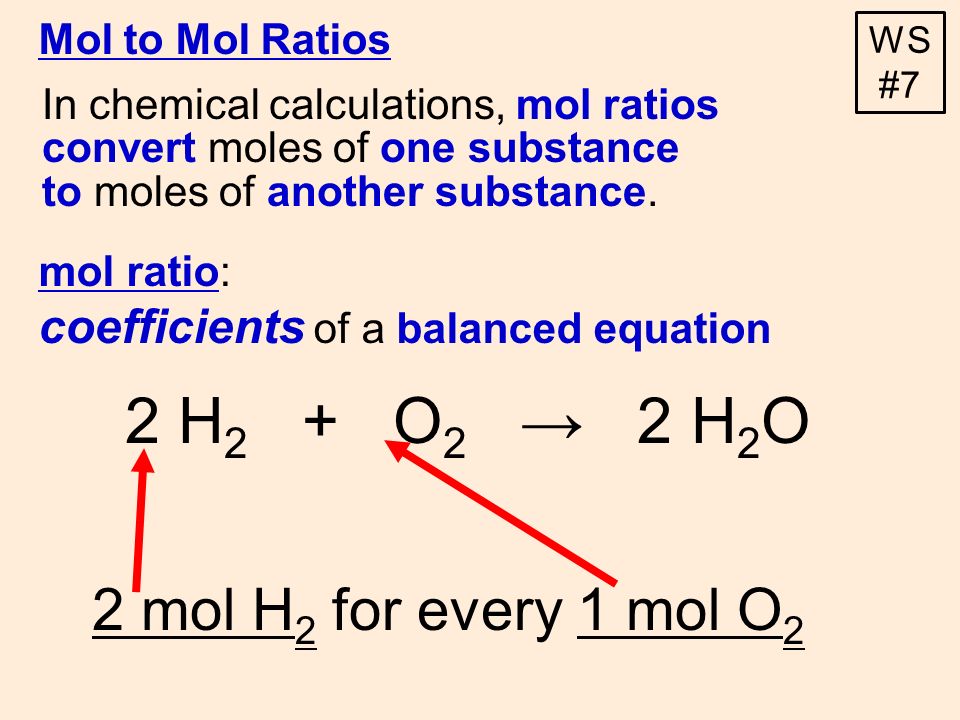
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

Practice Problem How many moles of aluminum oxide will be produced from 0.50 mol of oxygen? 4 Al + 3 O 2 → 2 Al 2 O mol? mol 3 O 2 = 2 Al 2 O ppt download
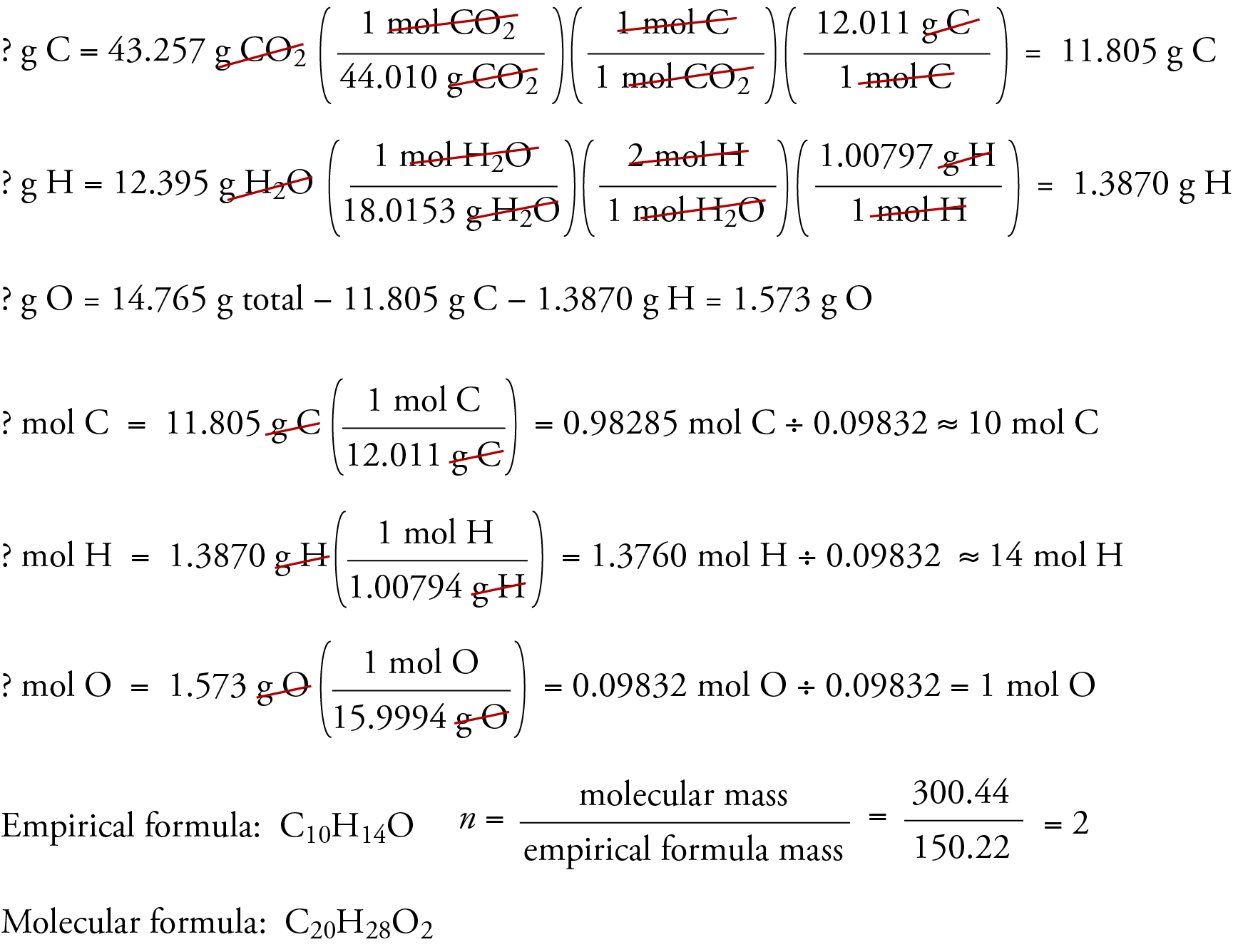
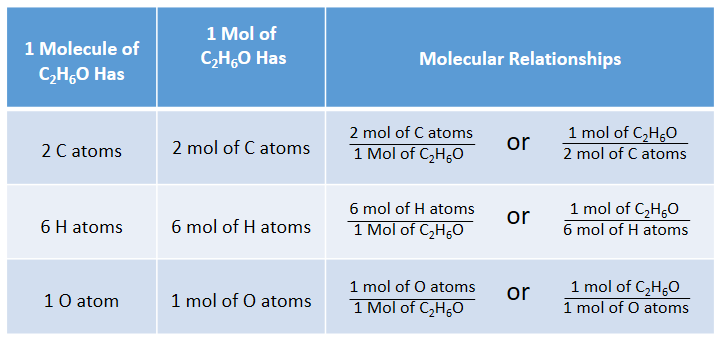
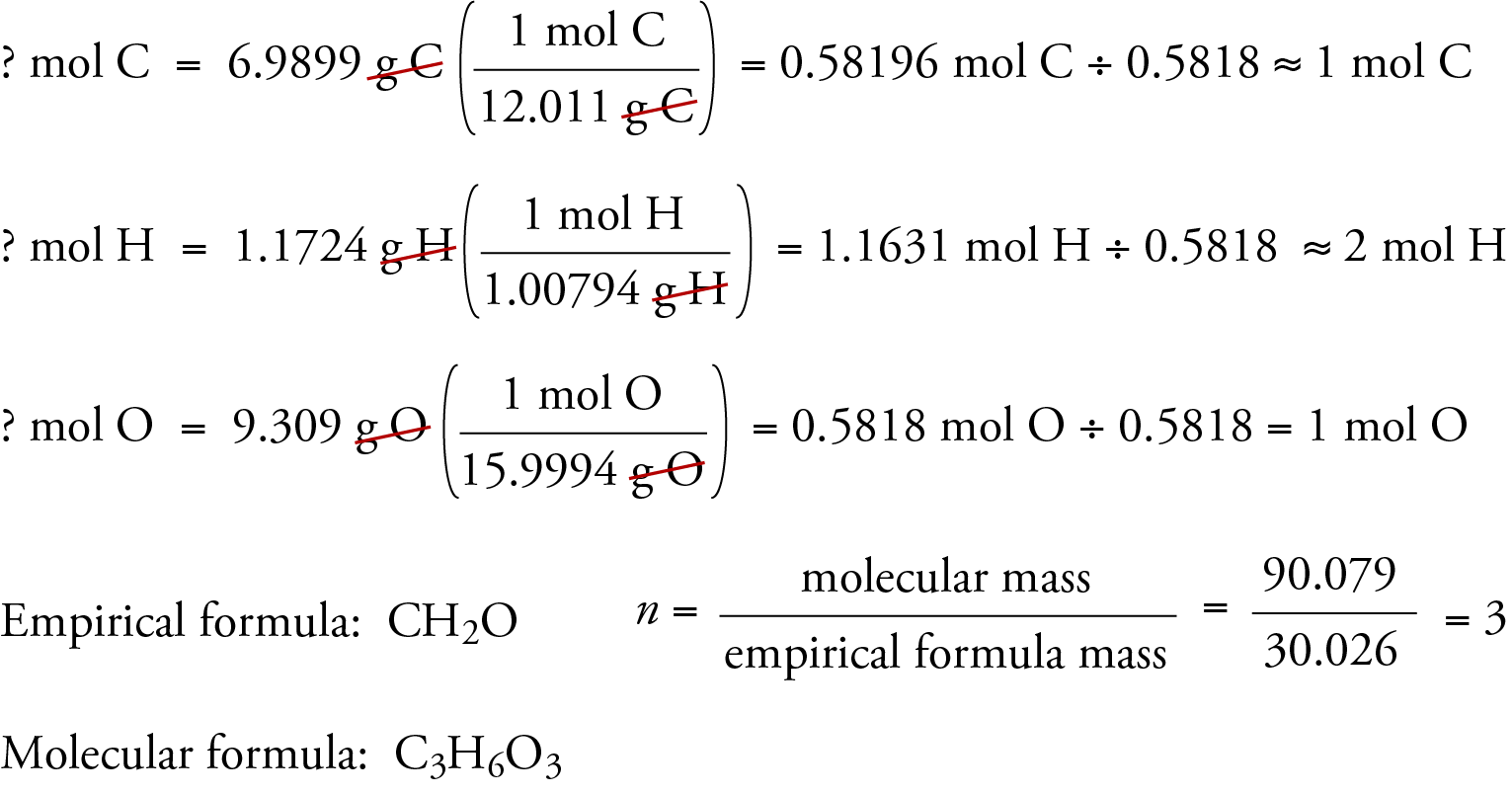
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

Grundlegende operationen der farbenchemie . gefrührt: Man löst 1 Molekül des Pnoles in 400 CMS Wasser,1 mol. Natronlauge et Soda, 80 g. Zu dieser Lösung gibtman 500 cm^ Alkohol (Methyl- bezw.
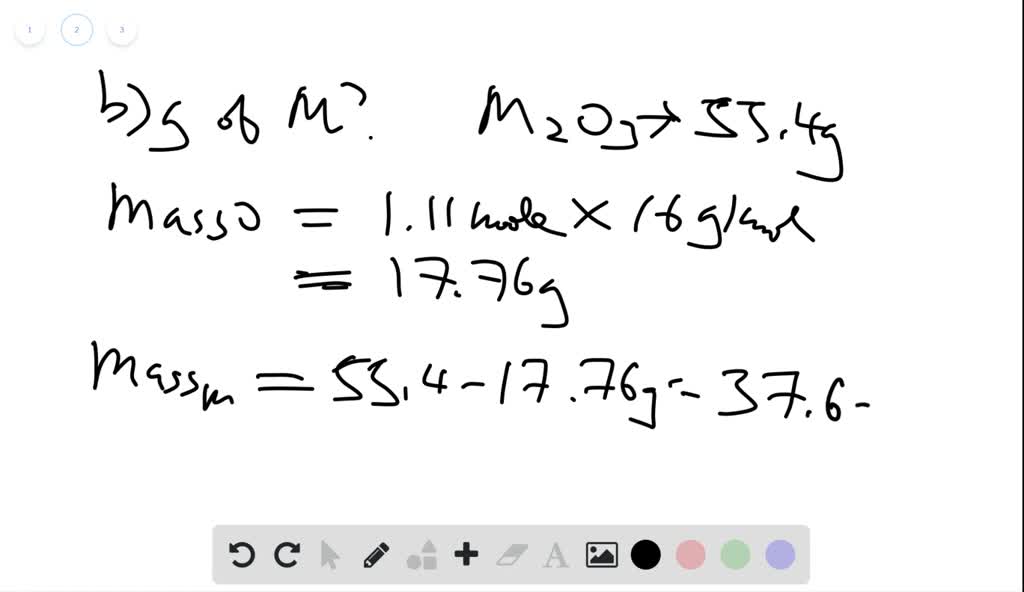
SOLVED: A 0.370-mol sample of a metal oxide (M2O3) weighs 55.4 g. (a) How many moles of O are in the sample? (b) How many grams of M are in the sample? (
:max_bytes(150000):strip_icc()/pancit-molo-recipe-5209963-hero-01-c89a8ced0df74edb99b4379d413fca58.jpg)