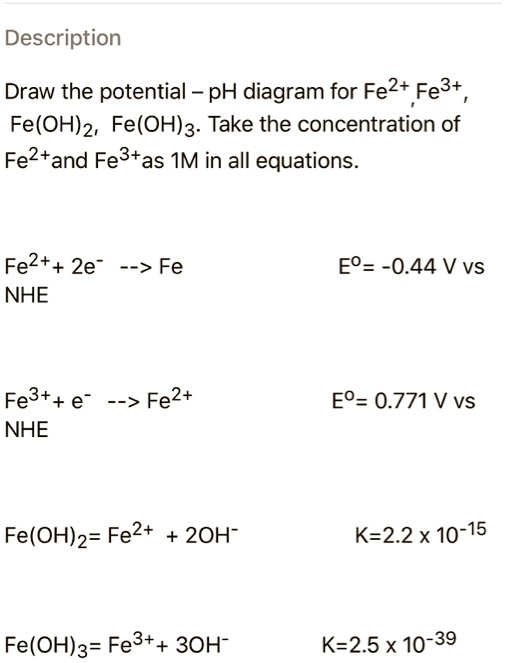
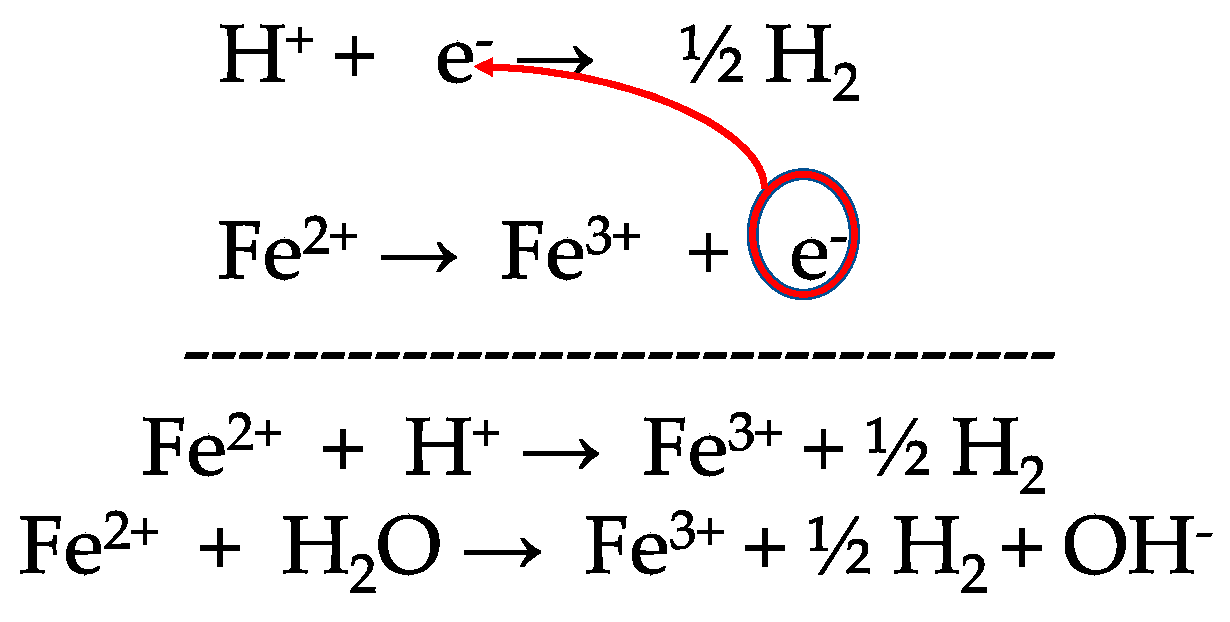Given standard electrode potentials, Fe^2 + 2e^-→ Fe, E^∘ = - 0.440 V Fe^3 + + 3e^-→ Fe, E^∘ = - 0.036 V The standard electrode potential (E^∘) for Fe^3 + + e^-→ Fe^2 + is:

Roles of Fe2+, Fe3+, and Cr3+ surface sites in the oxidation of NO on the (Fe,Cr)3O4(1 1 1) surface termination of an α-(Fe,Cr)2O3(0 0 0 1) mixed oxide - ScienceDirect

What is the standard reduction potential (E^o) for Fe^3 + → Fe ?Given that: Fe^2 + + 2e^ - → Fe ; E^oFe^2 + /Fe = - 0.47 V Fe^3 + + e^ - → Fe^2 + ; E^oFe^3/Fe^2 + = + 0.77 V

Color online) Energetic positions of Fe (Fe1, Fe2, and Fe3) and B (B... | Download Scientific Diagram

Enhanced electro-reduction of Fe3+ to Fe2+ by acidified carbon nanotube-modified graphite cathode and its application in a novel Fenton process for p-nitrophenol degradation - ScienceDirect

Double-enzymes-mediated Fe2+/Fe3+ conversion as magnetic relaxation switch for pesticide residues sensing - ScienceDirect

Schematic of intestinal iron uptake. Fe3+ in the intestinal lumen is... | Download Scientific Diagram

Exploring selective recognition between Fe2+, Fe3+ and their implementation in bio-imaging: A combined spectroscopic and theoretical investigation - ScienceDirect
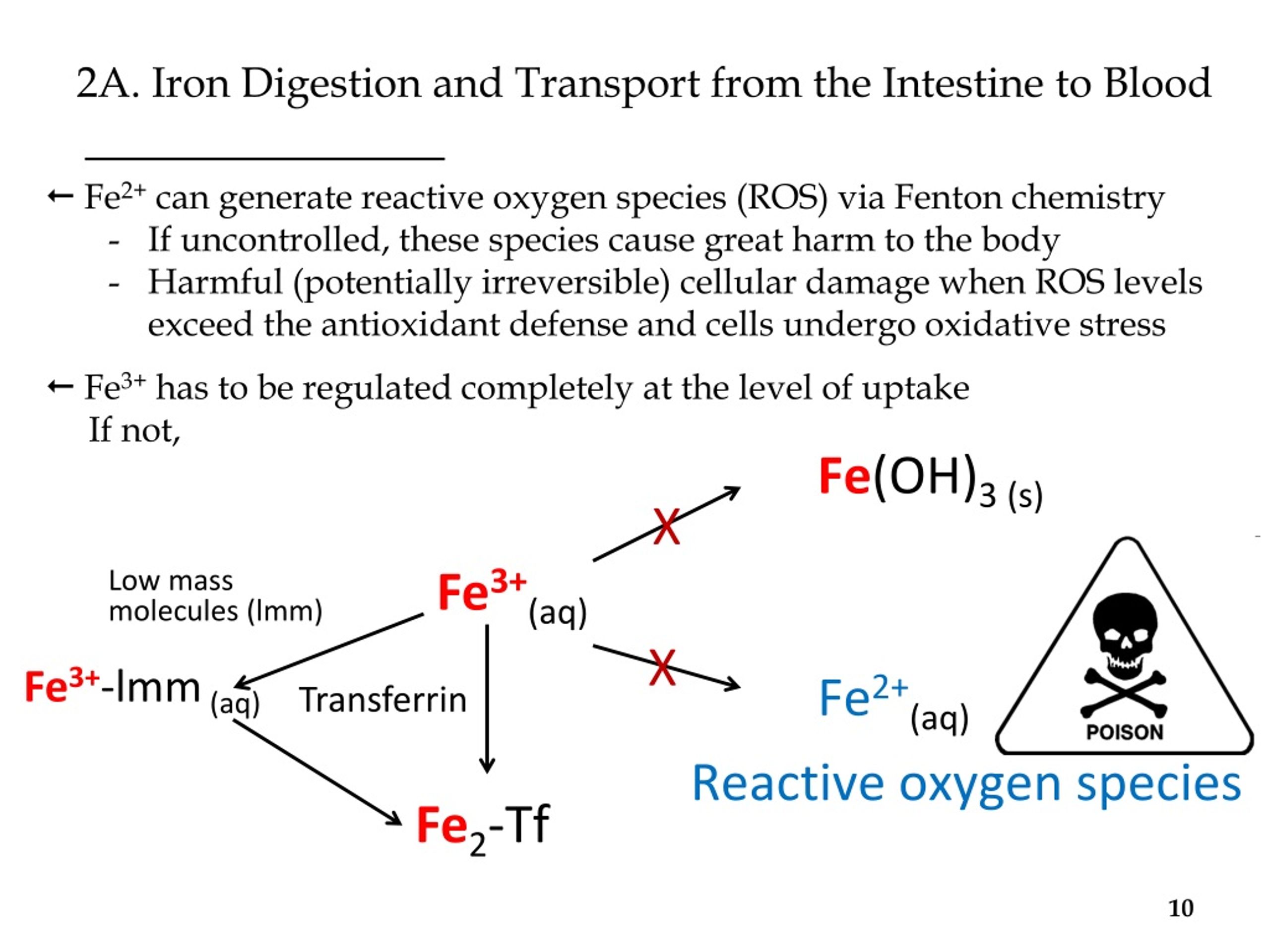
PPT - Iron (Fe 2+ /Fe 3+ ) Transport and Trafficking in Mammals Bertini et al Ch. 5 and 8 PowerPoint Presentation - ID:432903

Coordination numbers of the Fe 3 + and Fe 2 + ions calculated for their... | Download Scientific Diagram

Fe2+/Fe3+ Cycling for Coupling Self‐Powered Hydrogen Evolution and Preparation of Electrode Catalysts - Chen - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Inhibition of Fe2+- and Fe3+- induced hydroxyl radical production by the iron-chelating drug deferiprone - ScienceDirect

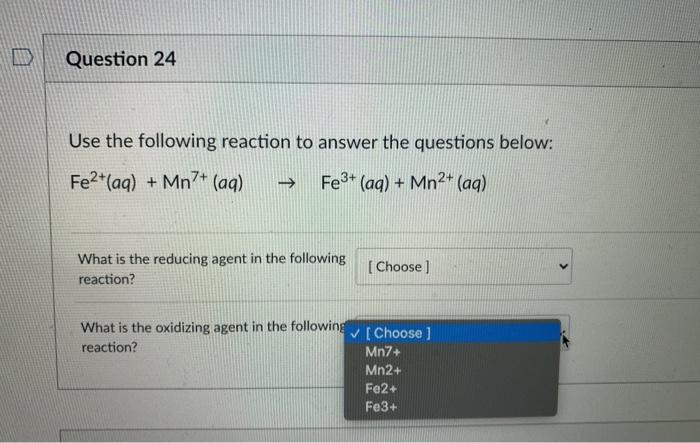
SOLVED: Consider the following voltaic cell: Pt(s)IFe2+ (aq) , Fe3+(aq) Ag" (aq)Ag(s) Identify the half-reaction that occurs at the anode Select one: a. Fe3- (aq) + e Fe2- (aq) b. Pt(s) +