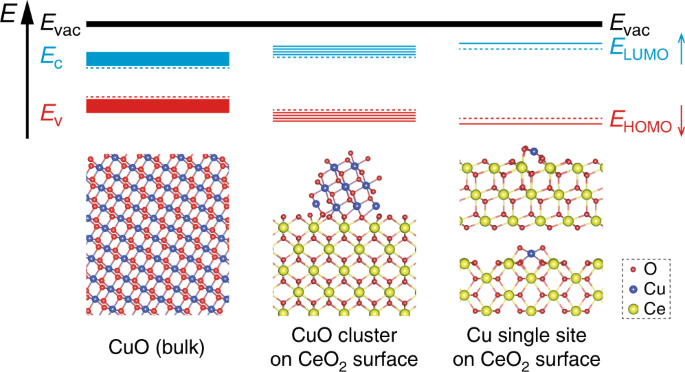
SFLPs on a) 0 mol% Cu-HAP (211) and b) 0.5 mol% Cu-HAP (211), where the... | Download Scientific Diagram

Applied Sciences | Free Full-Text | Molecular Dynamics Simulation of Bulk Cu Material under Various Factors

Thermodynamic calculation for the composition Cu 12 mol, S 13 mol, As 4... | Download Scientific Diagram

SOLVED: What is the volume V of a sample of 2.00 mol of copper? The atomic mass of copper (Cu) is 63.5 g/mol, and the density of copper is 8.92×103kg/m3. express the

Mole Conversions Practice.pptx - Convert 4 moles of Cu(CN)2 to grams 1 mole Cu(CN)2 = 63.5 Cu 24.0 C (12.0 x 2) 28.0 N (14.0 x 2) 115.5 g 4 mole | Course Hero