Selective Focus of Sodium Hydroxide Base and Sulfuric Acid Solution in Brown Glass and Plastic Bottle Stock Image - Image of harmful, class: 195465085

Acid – Base Reaction. Chemical Reaction Neutralization The Acid And Base Properties, Producing A Salt And Water.
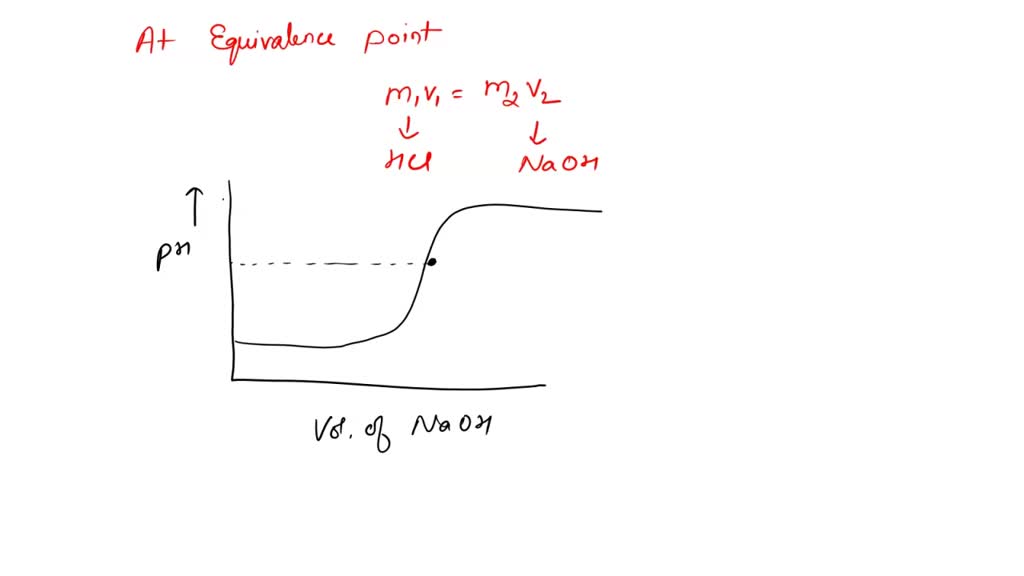
SOLVED: during titration of strong acid HCl and strong base NaOH, from the titration curve we get the ph at equivalence point is 7 and the volume of NaOH added is 25.1

Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution?

Effect of base (NaOH) and acid (HCl) additions on changes in buffering... | Download Scientific Diagram
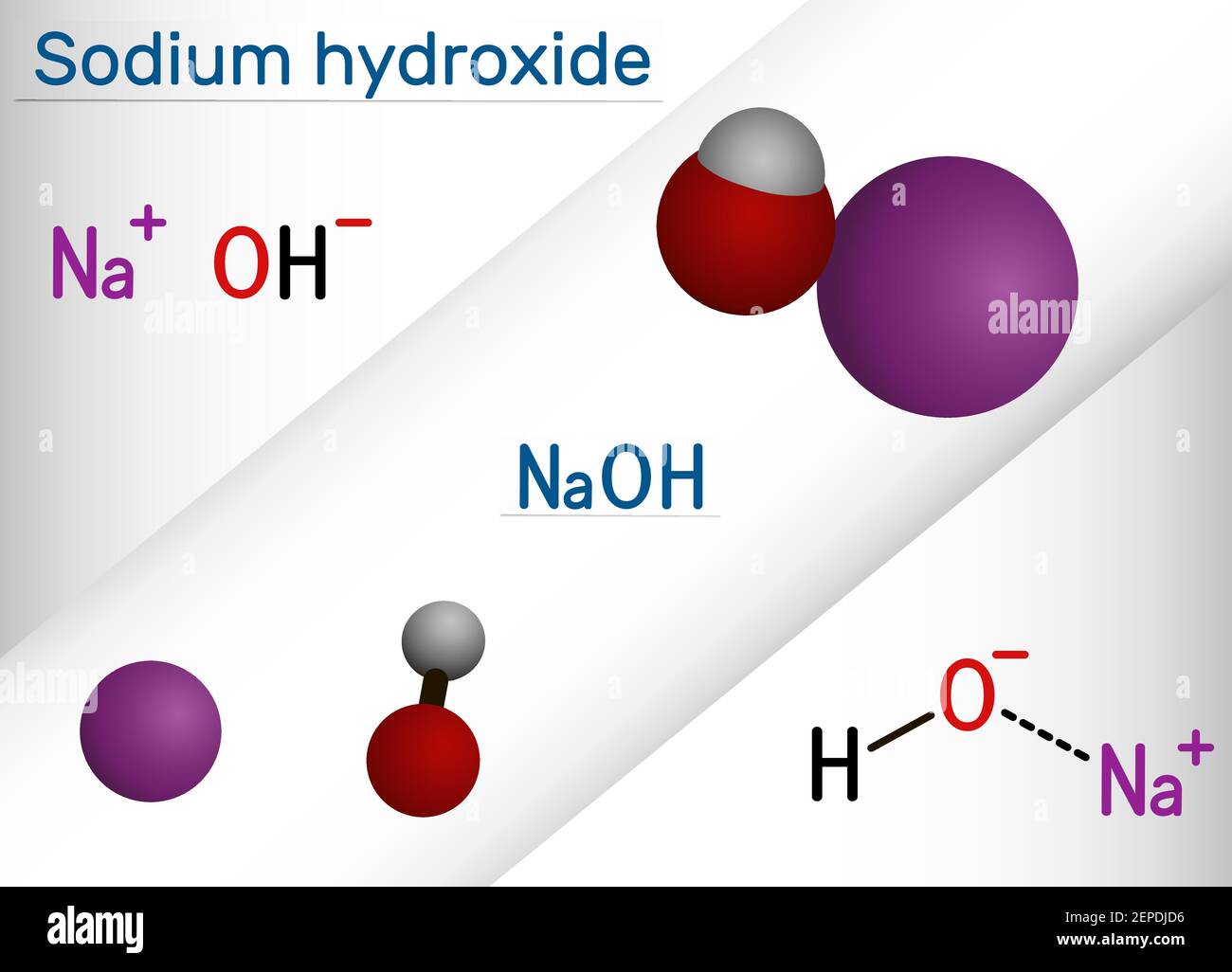
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy

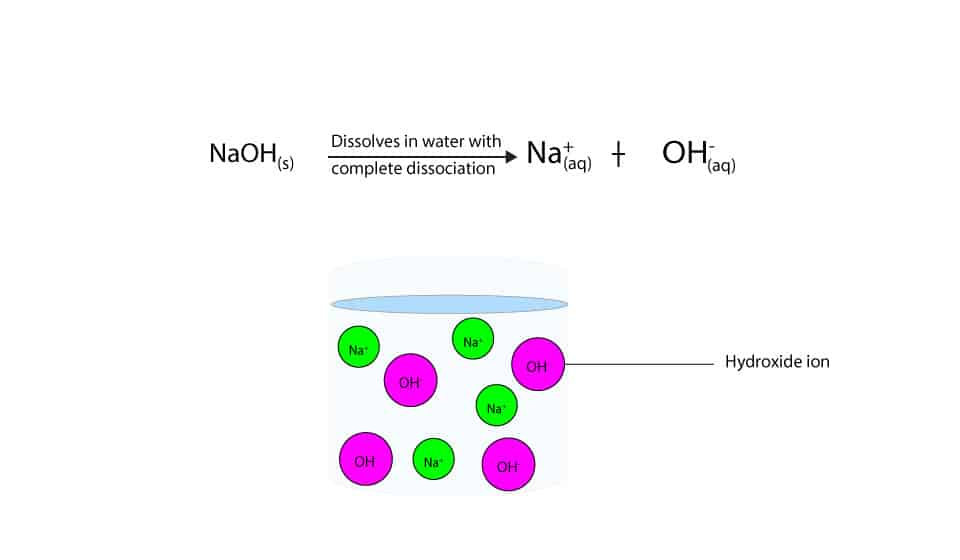

-in-water-01.jpg)